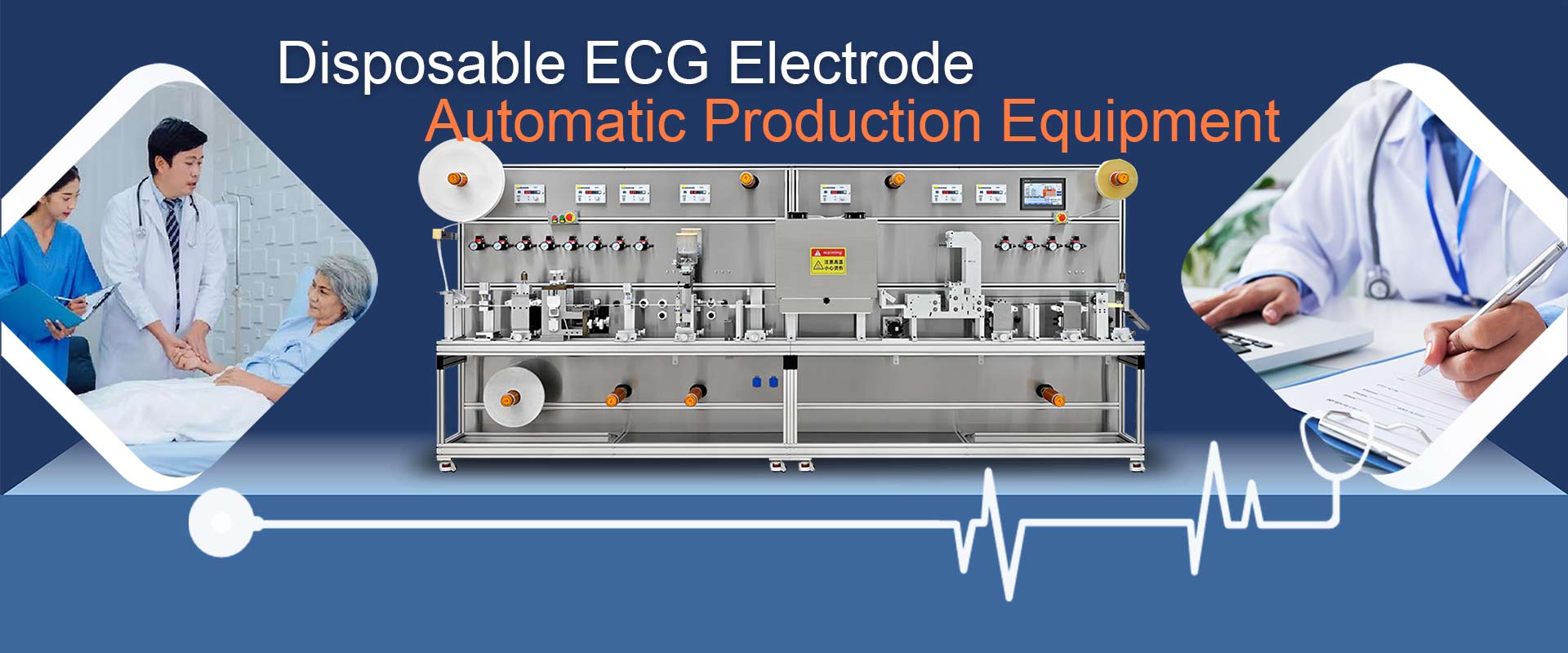
Disposable ECG Electrode
Automatic Production Equipment
The equipment is stable and easy to operate, with a production capacity of about 110 pieces (pcs) per minute. It can realize the interchange of various substrates. The knife molds of different shapes can be replaced according to the different shapes of the products. The electrode buckles are conductively tested. Unqualified products will not drip glue or fail. Forming, automatic waste discharge, automatic alarm and shutdown for material shortage/outage.
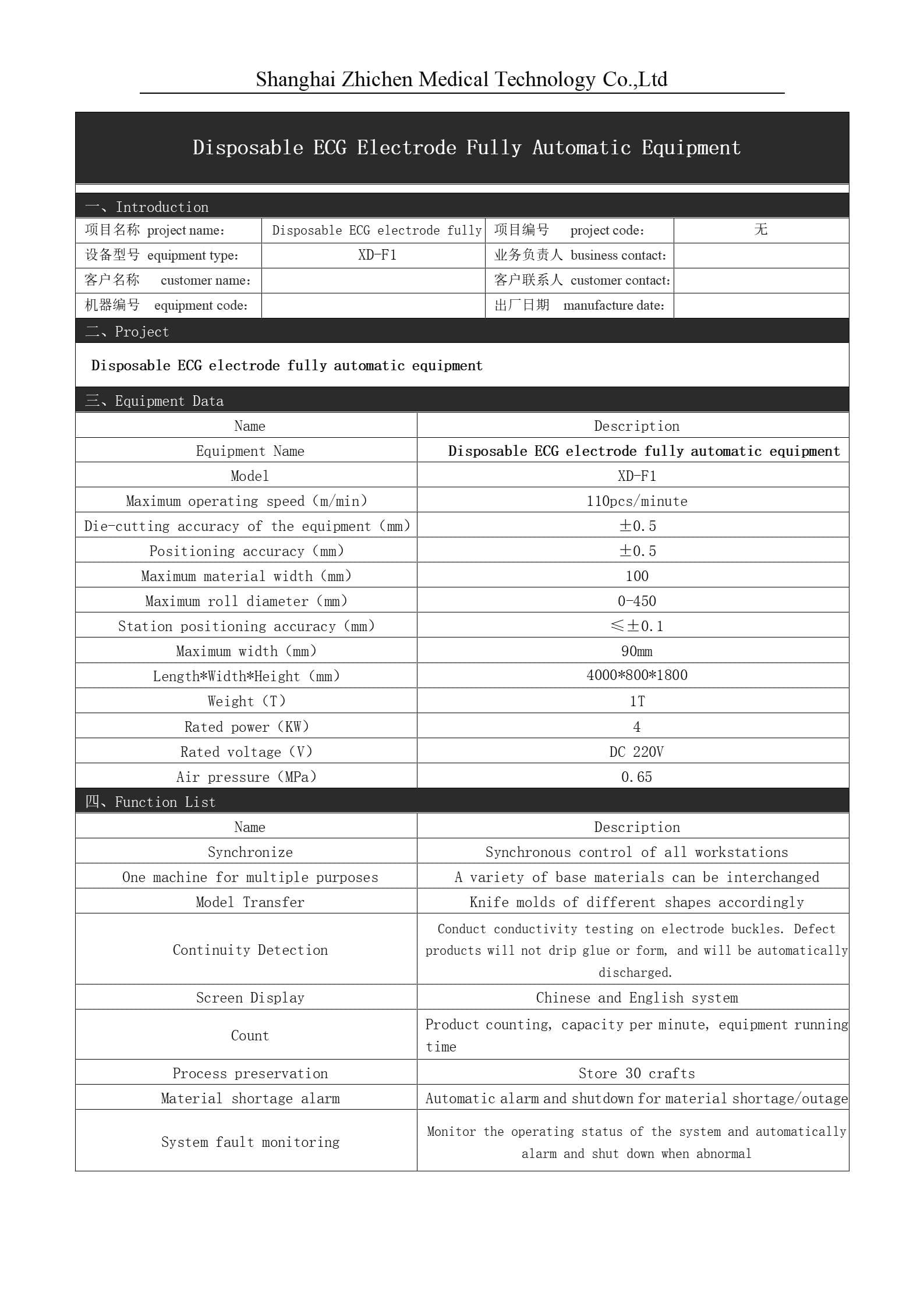
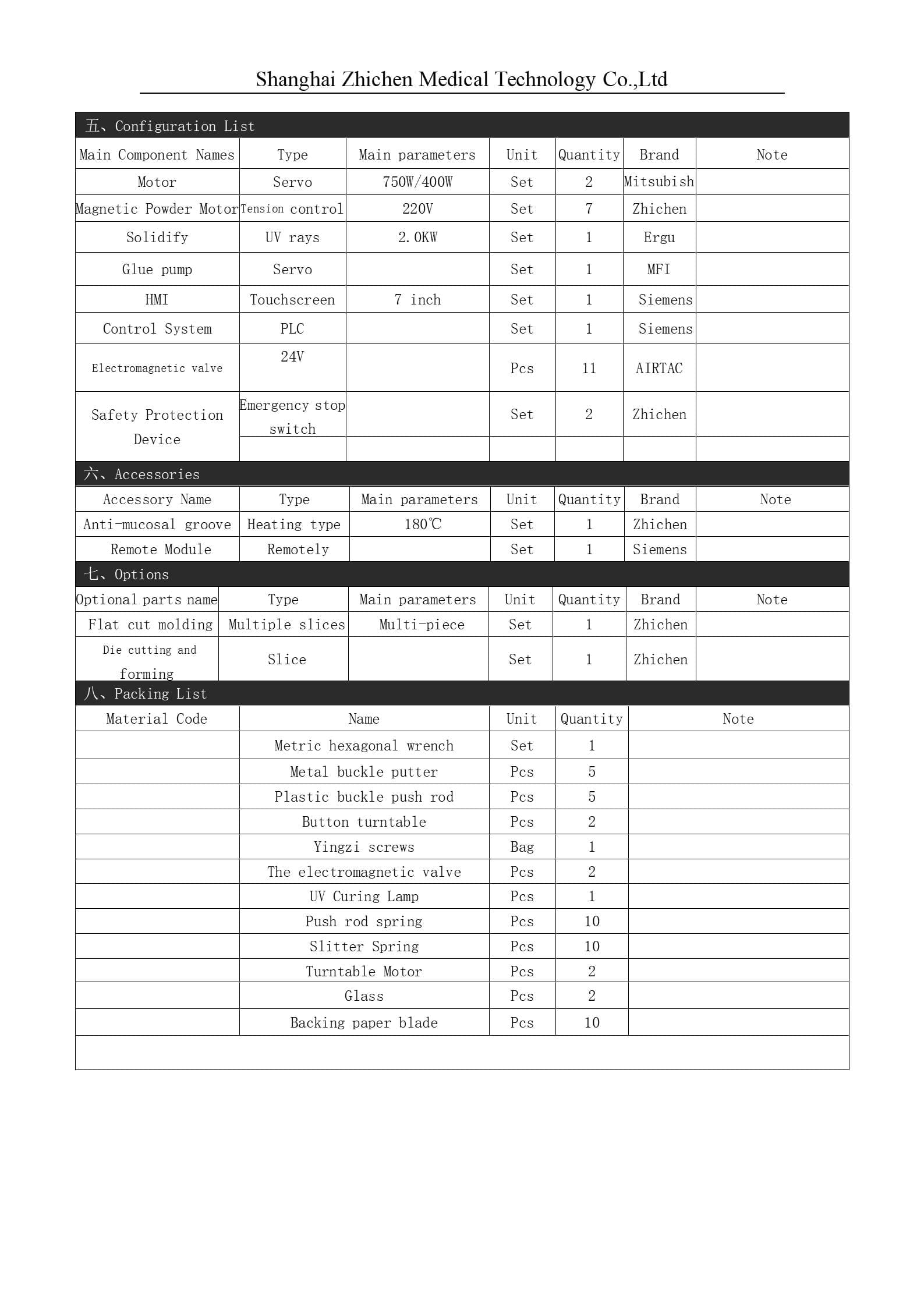
Disposable ECG Electrode User Manual
Product Name: Disposable ECG Electrode
Manufacturer License Number: Shanghai Pharmaceutical and Medical Device Production Permit No. 20233228
Product Registration Number: Shanghai Medical Device Registration No. 20232070156
Product Technical Requirements Number: Shanghai Medical Device Registration No. 20232070156
I.Scope of Application
This product is designed for use in conjunction with ECG collection equipment to collect and acquire surface ECG signals from individuals in medical institutions.
II.Performance Structure
This product is a disposable pre-gelled electrode.
The product consists of a backing material (gel-coated sponge/non-woven fabric), conductive gel, plastic fastener, metal fastener, and anti-adhesive membrane.
III. Main Specifications
AC Impedance: The impedance between two gel-to-gel electrode connections should be ≤3000Ω.
DC Offset Voltage: The DC offset voltage between electrode pairs should be ≤100mV.
Composite Offset Instability and Internal Noise: Should not exceed 150μV (peak-to-peak).
Defibrillation Overload Recovery: The absolute value of the polarization electromotive force should not exceed 100mV, with an average rate of change not exceeding ±1mV/s every 10 seconds. The AC impedance between electrode pairs should be ≤3000Ω after defibrillation.
Bias Current Tolerance: Within 8 hours, the voltage change between electrode pairs should be ≤100mV.
Bacterial Colony Count: ≤200 cfu/g; Fungal Colony Count: ≤100 cfu/g; No detection of coliforms or pathogenic pyogenic bacteria.
IV. Instructions for Use
When necessary, remove hair, clean the skin, and ensure it is dry.
Wipe the application site without damaging or injuring the skin.
Open the packaging and remove the backing material from the back.
Apply the electrode to the test location, ensuring that the electrode’s contact surface adheres securely to the skin.
Connect the linking wire from the associated equipment to the button above the electrode.
V. Precautions
Do not open the packaging unless you intend to use the electrode immediately.
Unused electrodes should be promptly returned to the packaging and sealed.
This product is for one-time use only.
Use with caution for individuals with allergies and the elderly.
Do not use the electrode on nipples, skin folds, bone structures, scar tissue, or inflamed areas.
Store this product in a well-ventilated indoor environment with a relative humidity not exceeding 80%.
VI.Manufacturing Date and Expiry Date
Please refer to the product packaging or outer box for the manufacturing date and expiry date.
VII. Quality Assurance
Store in an indoor environment with relative humidity not exceeding 80%, free from corrosive gases, and well-ventilated.
[Expiry Date] 24 months from the date of production, as specified on the outer packaging.

